Electroplating experiment aim to find the amount copper gains or loses on the electrodes using different amounts of current each time during electrolysis. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper.
 An Easy Copper Electroplating Demo For Your Redox Unit Chemical Education Xchange
An Easy Copper Electroplating Demo For Your Redox Unit Chemical Education Xchange
Conduct copper plating activity.

Electroplating lab experiment. Introduction electroplating is generally carried out in order to improve the. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability.
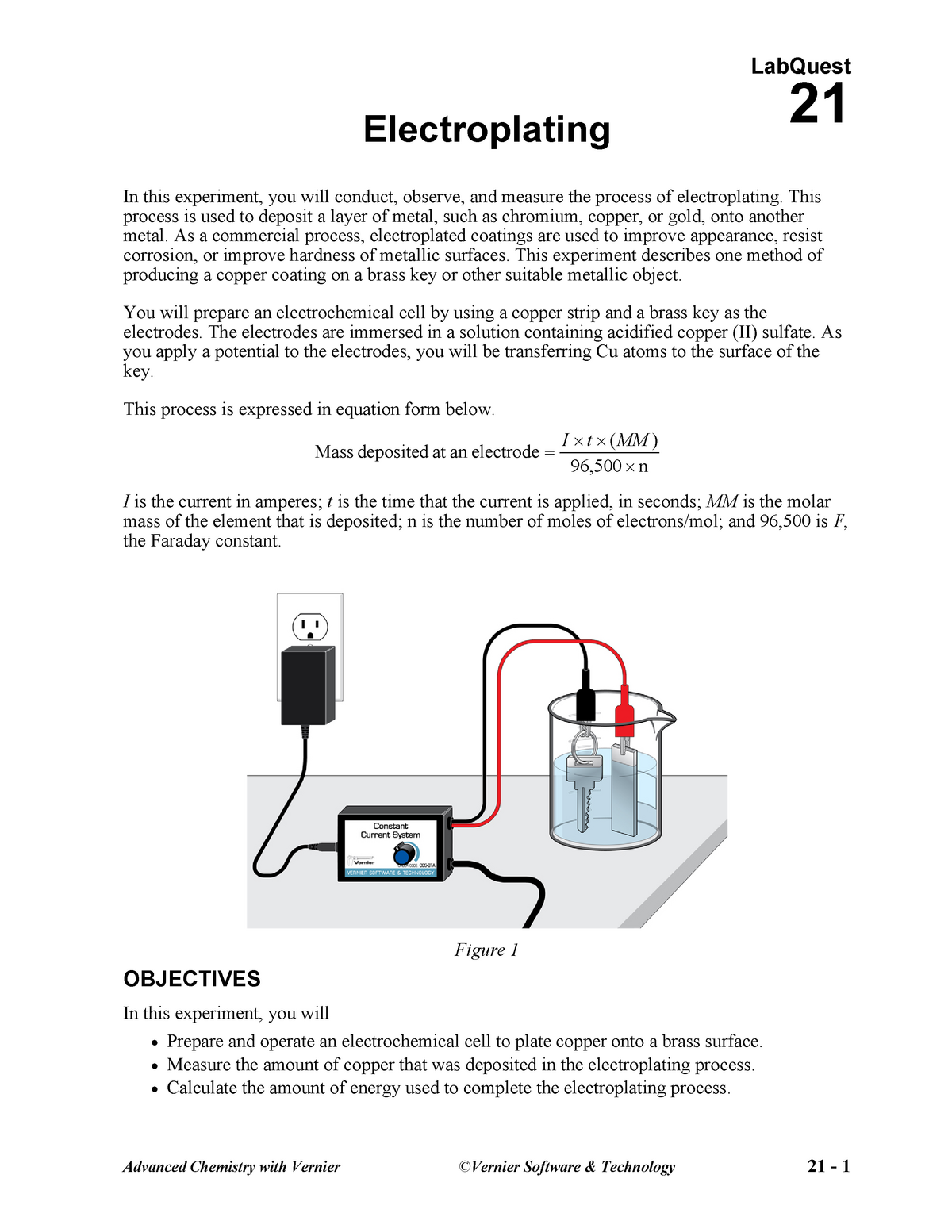
In our experiment. As a commercial process electroplated coatings are used to improve appearance resist corrosion or improve hardness of metallic surfaces. Electroplating is an energy intensive process.
Watch this video to see the electroplating of a copper key an electrolysis of water experiment and an electroplating experiment electrolysis. In this activity knowledge is crucial to complete the experiment. The twelve unknown puzzle.
During electroplating a thin layer of a desirable metal is deposited onto another object. The scientific goal of this experiment is to determine the efficiency of copper. Anion identification virtual lab.
In this lab exercise we consider the possibility of using a cheaper metal that has been electroplated with a thin layer of metallic copper that is subsequently treated to produce a light green patina. Determining the ksp of calcium hydroxide. This process is used to deposit a layer of metal such as chromium copper or gold onto another metal.
How the changing of current affects the electroplating of copper. For this experiment you can gather your own supplies or buy a complete water electrolysis kit. Post test 10 min game.
6 volt or 9 volt battery. In this experiment you will conduct observe and measure the process of electroplating. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current.
Play electrolysis walking tag suggested interpretation of the proverb. This lab is designed to provide an example of the reduction oxidation redox processes. Without knowledge you will be blind to what s out there and you will not know how to proceed.
Electroplating is an economically important process often used to reduce corrosion or improve the appearance of. Electrolysis and electroplating lab 4 chem 36 spring 2009 1 introduction reactions that involve a change in the number of electrons at a particular location require a reduction or oxidation process to take place. If the wires are misconnected.

 Lab Report11 Lab Report 11 Experiment Electroplating And The Measurement Of Avogadro S Number Prepared By Marat Seitimov Section 538 Date Perfomed Course Hero
Lab Report11 Lab Report 11 Experiment Electroplating And The Measurement Of Avogadro S Number Prepared By Marat Seitimov Section 538 Date Perfomed Course Hero
An Experiment To Show How Electroplating Using Copper Electrodes Gcse Science Marked By Teachers Com
Electroplating Lab Report College Homework Help And Online Tutoring
 Electroplating Ap Lab 21 Manualzz
Electroplating Ap Lab 21 Manualzz
 Electroplating Lab General Chemistry Studocu
Electroplating Lab General Chemistry Studocu
 Experiment C29 Electroplating Faraday S Law Of Electrolysis
Experiment C29 Electroplating Faraday S Law Of Electrolysis
 Experiment 25 Electroplating Ap Chem Lab Book 10 11 Of Brad Hekman
Experiment 25 Electroplating Ap Chem Lab Book 10 11 Of Brad Hekman
 Solved Logger Pro 21 Electroplating In This Experiment Y Chegg Com
Solved Logger Pro 21 Electroplating In This Experiment Y Chegg Com
 Lab 22 Electroplating Nickel Tri
Lab 22 Electroplating Nickel Tri

 Electroplating Experiment For Fun Paksc Org Electroplating Diy Electroplating Chemistry Experiments For Kids
Electroplating Experiment For Fun Paksc Org Electroplating Diy Electroplating Chemistry Experiments For Kids
-
Most beakers have spouts on their rims to aid in pouring. It is made of wood polythene or steel on which test tubes can be placed in an upr...
-
A titration is a very commonly used type of quantitative analysis. The titration apparatus as defined in claim 2 wherein. Titration Of Su...
-
One atom is present in each of the elements hydrogen carbon and nitrogen respectively. This formula points out that in one molecule of the ...
dark and stormy origin
Dark 'n' Stormy: The history of Bermuda's unofficial drink CNN . Bermuda, where the Dark ‘n’ Stormy was invented, is known...

ads

