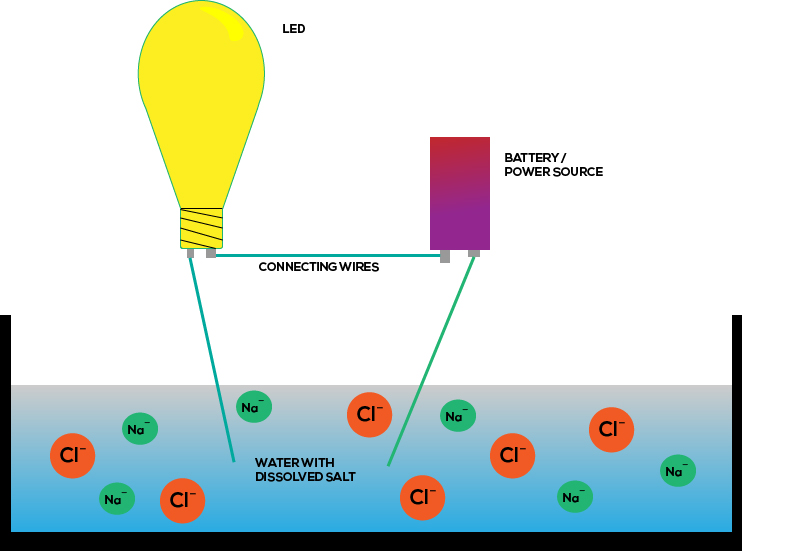
Salt is an ionic compound which is made up of sodium ions and chlorine ions. Its conductivity is greater than normal water.
 Ions In Water And Conductivity Laqua Water Quality Analyzer Website Horiba
Ions In Water And Conductivity Laqua Water Quality Analyzer Website Horiba
Meanwhile the semiconductor and pharmaceutical industries require extremely pure water with an even lower electrical conductivity value than drinking water.

Salt water electrical conductivity. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Conductivity is traditionally determined by connecting the electrolyte in a wheatstone bridge.
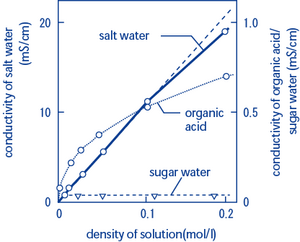
Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. Dissolving solid sodium chloride table salt in water releases ions according to the equation in this experiment you will study the effect on electrical conductivity of increasing sodium chloride concentration.
As an example the electrical conductivity of drinking water will usually be less than 1 ms cm. The conductivity of pure water used in such applications is typically less than 1 µs cm. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge when you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely.
Why does salt water conduct electricity. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
The major positively charged ions are sodium na calcium ca 2 potassium k and magnesium mg 2. The saltwater is a good conductor of electricity. If an ionic solid is dissolved in water ions are released and the resulting solution will conduct electricity.
Salt molecules are made of sodium ions and chlorine ions. Salts that dissolve in water break into positively and negatively charged ions. This is because salt molecules are made of sodium ions and chlorine ions nacl.
High quality deionized water has a conductivity of about 0 5 μs cm at 25 c typical drinking water is in the range of 200 800 μs cm while sea water is about 50 ms cm or 50 000 μs cm. Roobert33 this experiment is only for vision it serves to understand how the salt dissolved in water facilitates an optimal condition for the passage of th.
 Shows The Location Of The Electrical Conductivity Sensors For Download Scientific Diagram
Shows The Location Of The Electrical Conductivity Sensors For Download Scientific Diagram
Conductivity Of Water Easy Science Experiment For Kids
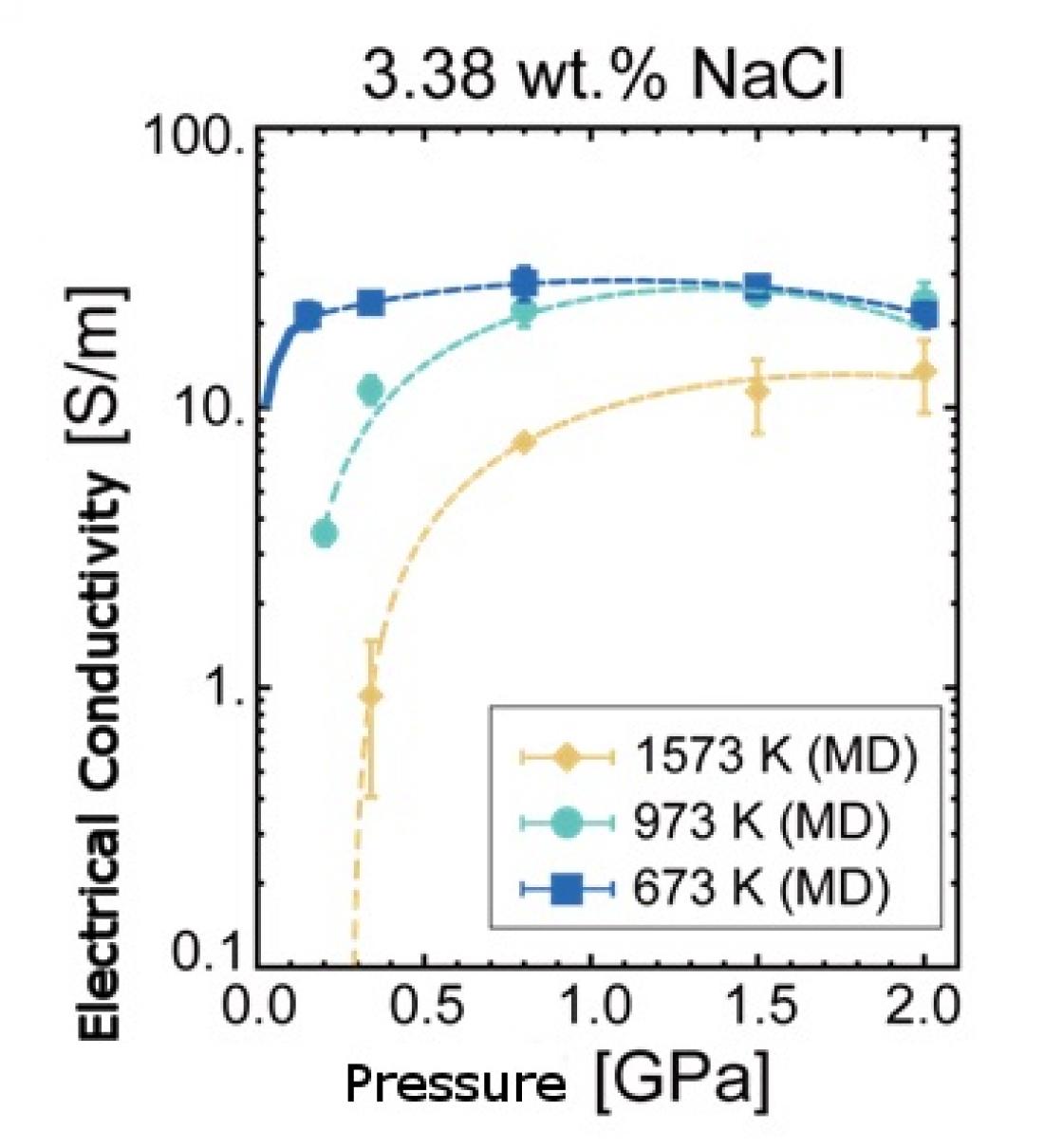 Electrical Conductivity Of Salt Water In Seismogenic Zones Theoretically Determined Asia Research News
Electrical Conductivity Of Salt Water In Seismogenic Zones Theoretically Determined Asia Research News
 Conductivity Of A Solution Andy Connelly
Conductivity Of A Solution Andy Connelly
 Conductivity Electrolytic Wikipedia
Conductivity Electrolytic Wikipedia
 Electrical Conductivity And Salinity Levels Of Fluids
Electrical Conductivity And Salinity Levels Of Fluids
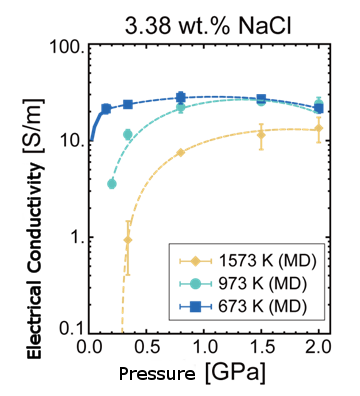 Electrical Conductivity Of Salt Water In Seismogenic Zones Theoretically Determined Nims
Electrical Conductivity Of Salt Water In Seismogenic Zones Theoretically Determined Nims
Conductivity Electrolytic Wikipedia
 Electrical Conductivity Of Salt Water Experiment Diy Youtube
Electrical Conductivity Of Salt Water Experiment Diy Youtube
 Salt Water Conductivity Experiment Middle School Science Experiments School Science Experiments School Science Projects
Salt Water Conductivity Experiment Middle School Science Experiments School Science Experiments School Science Projects
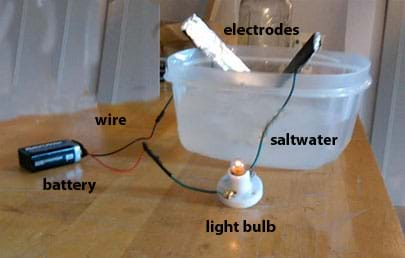 Saltwater Circuit Activity Teachengineering
Saltwater Circuit Activity Teachengineering
 Electrical Conductivity With Salt Water Working Model School Science Exhibition Project Youtube
Electrical Conductivity With Salt Water Working Model School Science Exhibition Project Youtube
 Electrical Conductivity With Salt Water Sugar Water Youtube
Electrical Conductivity With Salt Water Sugar Water Youtube
 Electrical Conductivity And Ph Of Soil As A Result Of Salt Accumulation Download Table
Electrical Conductivity And Ph Of Soil As A Result Of Salt Accumulation Download Table
-
Most beakers have spouts on their rims to aid in pouring. It is made of wood polythene or steel on which test tubes can be placed in an upr...
-
A titration is a very commonly used type of quantitative analysis. The titration apparatus as defined in claim 2 wherein. Titration Of Su...
-
One atom is present in each of the elements hydrogen carbon and nitrogen respectively. This formula points out that in one molecule of the ...
dark and stormy origin
Dark 'n' Stormy: The history of Bermuda's unofficial drink CNN . Bermuda, where the Dark ‘n’ Stormy was invented, is known...

ads
