C 3 h 8 190 42. Therefore molecular compounds usually have low melting and boiling points.
 Chapter 2 Section 2 2 Molecular Compounds Molecular Element Molecular Compound Diatomic Made Up Of Two Atoms Molecular Element Consists Of Molecules Ppt Download
Chapter 2 Section 2 2 Molecular Compounds Molecular Element Molecular Compound Diatomic Made Up Of Two Atoms Molecular Element Consists Of Molecules Ppt Download
The enthalpy of fusion is the amount of energy needed at constant pressure to melt one mole of a solid substance.

Physical properties of molecular compounds. C 5 h 12 130. The covalent bonds in such compounds are flexible and bend or break easily. List two general properties of molecular compounds.
C 2 h 6 183 89. Ch 4 182 164. These vary dramatically from substance to substance even for substances which appear similar in molecular formulae with some melting temperatures in the hundreds or thousands of degrees celsius and others well below.
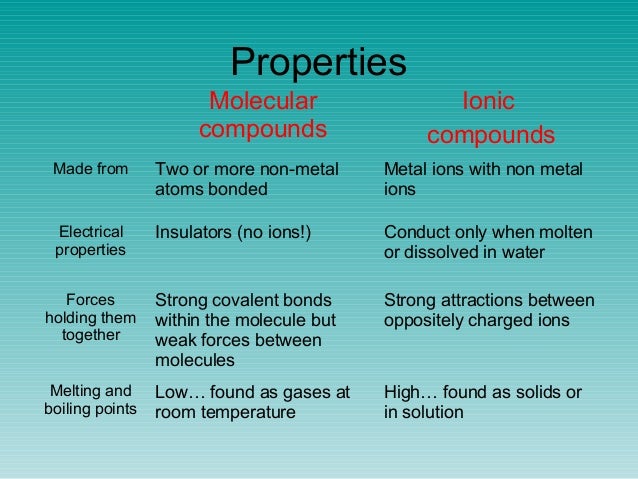
Low melting points and boiling points. Properties of simple molecular substances the physical properties of simple molecular substances can be explained by thinking about their structure and bonding. Simple examples of physical properties which can be related to molecular properties are the melting and boiling temperatures.
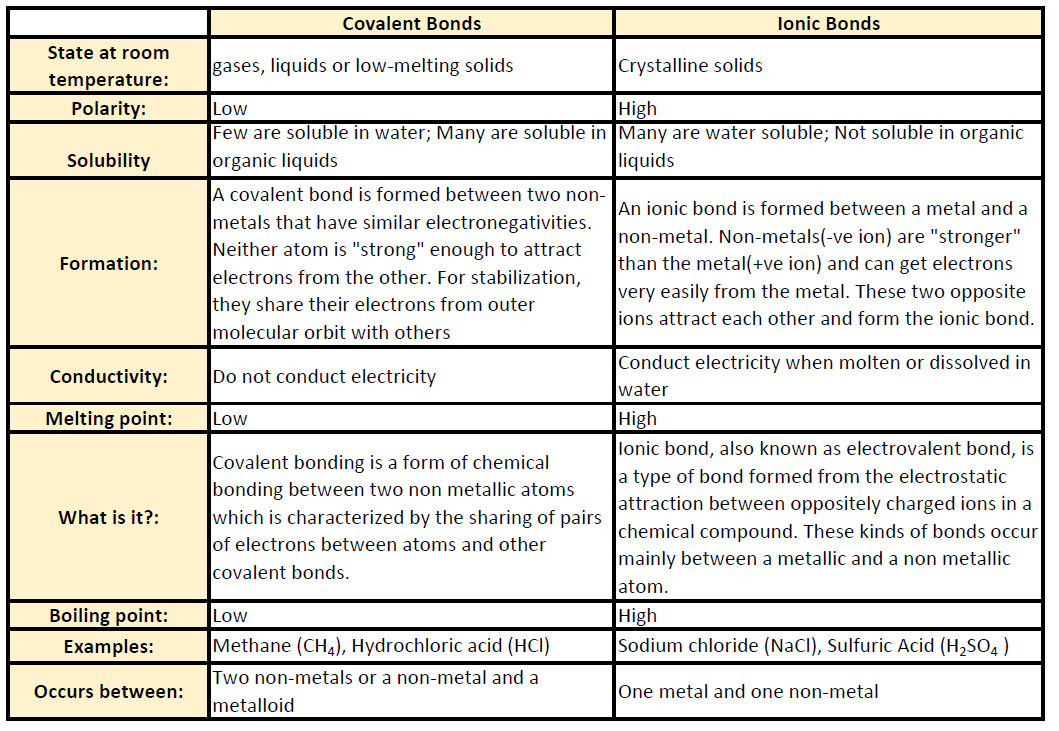
Molecular name formula melting point c boiling point c density at 20 c physical state at 20 c methane. The physical properties of molecular substances molecules are made of fixed numbers of atoms joined together by covalent bonds and can range from the very small even down to single atoms as in the noble gases to the very large as in polymers proteins or even dna. Several physical properties of molecules compounds are related to the presence of covalent bonds.
Molecular compounds sometimes called covalent compounds display a wide range of physical properties due to the different types of intermolecular attractions such as different kinds of polar interactions. Physical properties of some alkanes. The specific optical rotation of aqueous and organic solutions of these compounds was measured and was in agreement with their stoichiometric formula.
The covalent bonds in such compounds are flexible and bend or break easily. What are three properties of molecular. C 4 h 10 138 1.
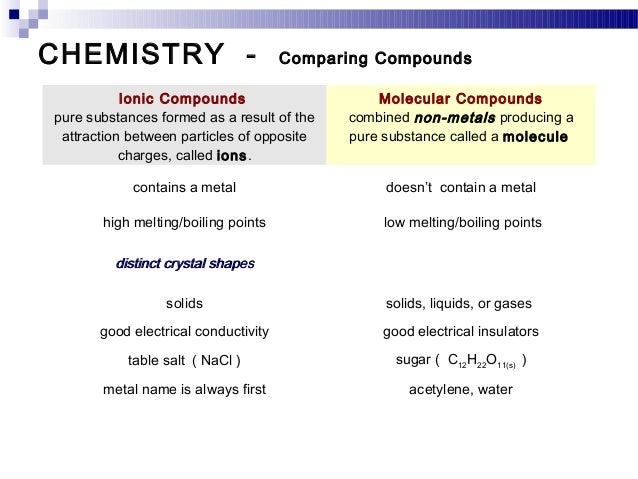
The solid forms of molecular compounds are generally very soft and brittle. Two general properties of molecular compounds are lower melting and boiling points than ionic compounds. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds.
Covalent bonds between atoms are quite strong but attractions between molecules compounds or intermolecular forces can be relatively weak. The physical and chemical properties of the molecular compounds of lactose 5α 3β and 3α 2β lactose were studied the melting point and the heat of combustion were determined. The melting and boiling points of molecular compounds are generally quite low compared to those of ionic compounds.
 Physical Properties And Molecular Formula Of The Synthesized Compounds Download Table
Physical Properties And Molecular Formula Of The Synthesized Compounds Download Table
 2 3 Molecular Compounds Bonding And Properties Lo I Understand How The Bonding Present Affects The Physical Properties Of A Molecular Substance Ppt Download
2 3 Molecular Compounds Bonding And Properties Lo I Understand How The Bonding Present Affects The Physical Properties Of A Molecular Substance Ppt Download
 Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
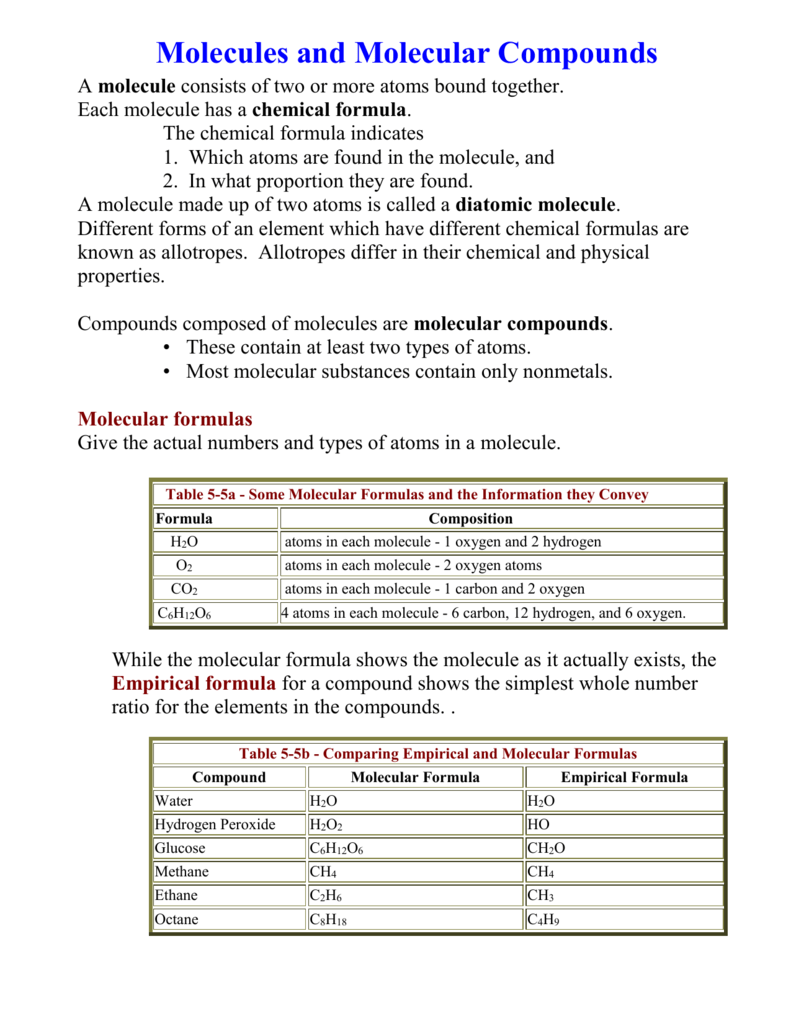 Molecules And Molecular Compounds
Molecules And Molecular Compounds
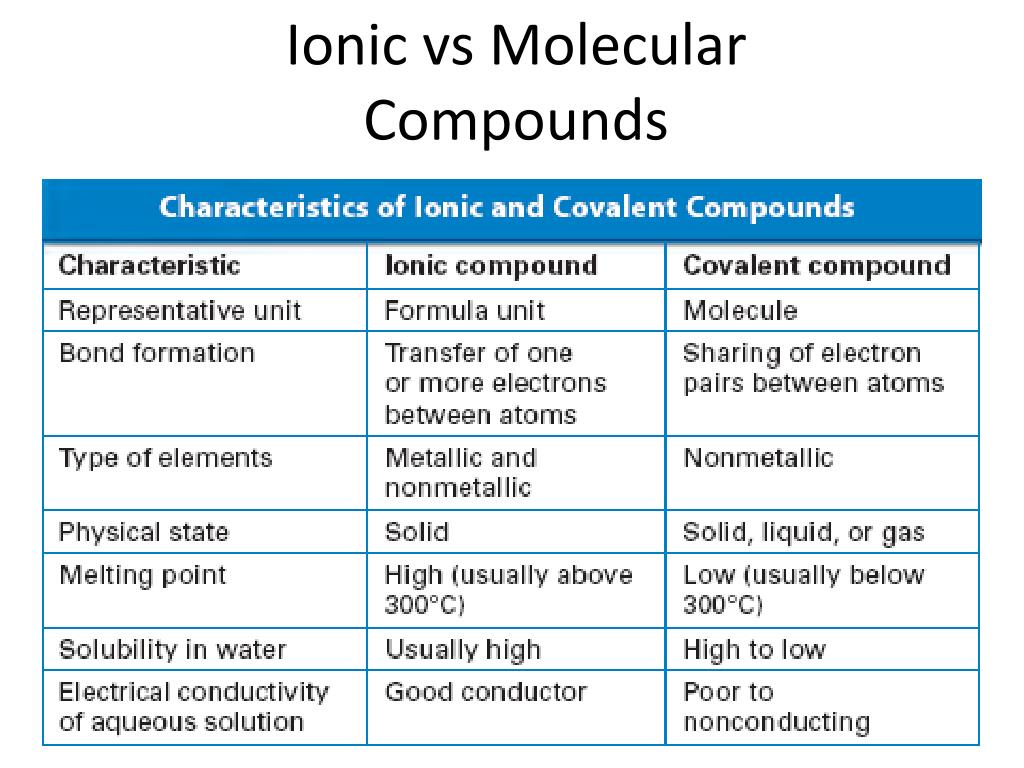
 Comparison Of Ionic And Covalent Compounds
Comparison Of Ionic And Covalent Compounds
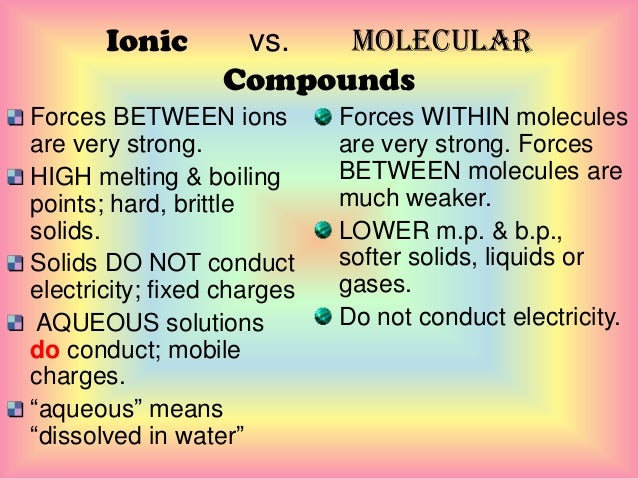 Properties Of Covalent Substances Metals And Ionic Compounds
Properties Of Covalent Substances Metals And Ionic Compounds
 Molecular Structure And Properties Of Compounds Chemistry 30 Unit Ppt Download
Molecular Structure And Properties Of Compounds Chemistry 30 Unit Ppt Download
 Difference Between Ionic And Molecular Compounds Infographic Chemistry Lessons Study Chemistry Teaching Chemistry
Difference Between Ionic And Molecular Compounds Infographic Chemistry Lessons Study Chemistry Teaching Chemistry
 Ppt Ionic Vs Molecular Compounds Powerpoint Presentation Free Download Id 3074480
Ppt Ionic Vs Molecular Compounds Powerpoint Presentation Free Download Id 3074480
 Physical Properties And Molecular Formulae Of The Synthesized Compounds Download Table
Physical Properties And Molecular Formulae Of The Synthesized Compounds Download Table
-
Most beakers have spouts on their rims to aid in pouring. It is made of wood polythene or steel on which test tubes can be placed in an upr...
-
A titration is a very commonly used type of quantitative analysis. The titration apparatus as defined in claim 2 wherein. Titration Of Su...
-
One atom is present in each of the elements hydrogen carbon and nitrogen respectively. This formula points out that in one molecule of the ...
dark and stormy origin
Dark 'n' Stormy: The history of Bermuda's unofficial drink CNN . Bermuda, where the Dark ‘n’ Stormy was invented, is known...

ads